VDRL stands for The Venereal Disease Research Laboratory, is a blood test used to screen for syphilis, a sexually transmitted infection caused by the bacterium Treponema pallidum. The test is based on the detection of antibodies produced by immune system in response to the infection.
It can also be used to monitor the effectiveness of treatment and to screen high risk individuals such as pregnant womens or individuals who engage in risky sexual behaviours. It can lead to serious health conditions if left untreated.
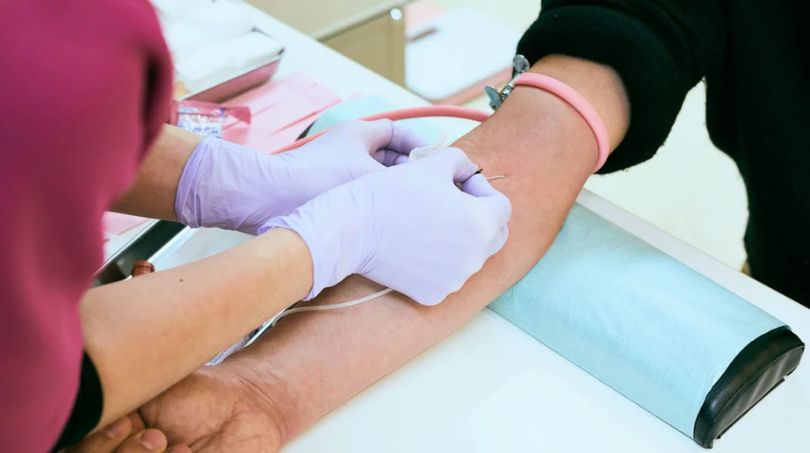
While the VDR is a valuable screening tool, it has some limitations. One such limitation is its reduced sensitivity during the early stage of syphilis. It takes time for the body to produce detachable levels of antibodies, which can result in false negative result. In such cases, additional testing methods like the Treponemal antibody test is conducted.
Another limitation is the possibility of the false positive results, such conditions like autoimmune diseases, recent vaccinations, pregnancy, or other infections, can lead to false-positive VDRL test results. Therefore, confirmatory testing is essential to rule out false positives and ensure accurate diagnosis.
Read more about- PET Scan test
About Test
About VDRL Test
When & Why
When & Why
The VDRL (Venereal Disease Research Laboratory) test is conducted in specific situations to screen, diagnose, or monitor syphilis infections. Here are some common scenarios when the VDRL test is conducted:
- Routine STI screenings: Healthcare providers often recommend STI screening test for sexually active individuals, especially who engage in high risk behaviour or have multiple sexual partners.
- Symptoms of Syphilis: When an individual presents with symptoms such as genital sores, rash or other signs of infection, the VDRL test is conducted to confirm the diagnosis.
- Pregnancy screening: Syphilis can be transmitted from a pregnant woman to her unborn child, leading to severe health complications. As part of routine prenatal care, the VDRL test is often performed to screen pregnant women for syphilis.
- Contact tracing: In situations where an individual has been in close contact with someone diagnosed with syphilis, contact tracing may be initiated. The VDRL test is conducted on these individuals to identify potential infections and prevent further transmission.
- Monitoring treatment effectiveness: After initiating treatment for syphilis, regular monitoring is essential to ensure the effectiveness of the prescribed therapy. The VDRL test is conducted at specific intervals during and after treatment to track changes in the levels of syphilis antibodies. A declining titer indicates a positive response to treatment.
- Preoperative screening: In certain surgical procedures, particularly those involving organ transplantation, blood transfusion, or immunosuppressive therapy, preoperative screening for syphilis may be required. The VDRL test helps identify any existing syphilis infection that may pose risks during the procedure.
Note: It’s important to note that the decision to conduct a VDRL test is ultimately made by a healthcare professional based on individual circumstances, risk factors, and clinical judgment. If you suspect you have been exposed to syphilis or have concerns about your sexual health, it’s best to consult a healthcare provider who can guide you through the appropriate testing process.
Test Procedure
Test Procedure
The VDRL (Venereal Disease Research Laboratory) test is a blood test used to screen for syphilis. Here is an overview of the test procedure:
- Blood sample collection: A healthcare professional will typically clean the area with an antiseptic, usually the inner elbow or the back of the hand, and then insert a needle into a vein to draw the blood. They may use a tourniquet to help locate a suitable vein.
- Sample processing: Once the blood sample is collected, it is sent to a laboratory for processing. In the lab, the blood is placed in a test tube and allowed to clot. After clotting, the sample is centrifuged to separate the serum, which contains the antibodies, from the rest of the blood components.
- Test Execution: The VDRL test utilizes a card or a plate coated with an antigen derived from the Treponema pallidum bacteria, the causative agent of syphilis. The serum from the blood sample is diluted and mixed with the antigen on the test card or plate. The mixture is then examined for visible reactions.
- Observation and interpretation: The VDRL test is read by visual inspection. A positive reaction is indicated by the presence of clumping or agglutination, which can be seen as irregular patterns or clumps within the test area. A negative reaction shows a smooth and even appearance across the test area.
- Titer determination: The titer indicates the dilution of the serum at which the positive reaction can still be observed. For example, a titer of 1:8 means that the serum can be diluted eight times and still produce a positive reaction.
Note: It’s important to note that the VDRL test is a screening test, and if the result is positive, it requires confirmation using additional tests, such as treponemal antibody tests like the FTA-ABS (Fluorescent Treponemal Antibody Absorption) or TP-PA (Treponema pallidum Particle Agglutination) test. These tests are more specific and can confirm the presence of syphilis antibodies.
Normal Range
Normal Range
The result of the VDRL test is reported as a titer, which indicates the dilution of the serum at which a positive reaction occurs. The titer is expressed as a ratio, such as 1:4, 1:8, 1:16, and so on.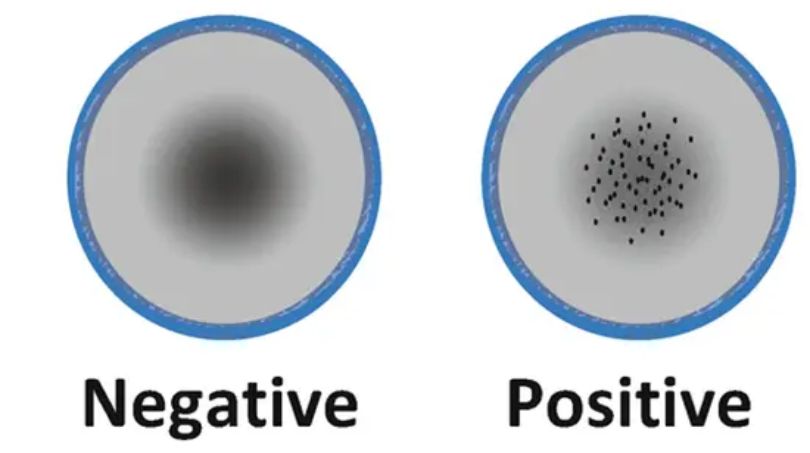
In general, a lower titer (e.g., 1:4) is considered a weaker or less significant reaction, while a higher titer (e.g., 1:64) is considered a stronger or more significant reaction. However, it’s important to note that the interpretation of VDRL test results depends on various factors, including the individual’s clinical history, the stage of syphilis (if present), and the presence of any confirmatory test results.
Results & Reports
Results & Reports
The results and reports of the VDRL (Venereal Disease Research Laboratory) test are typically presented in a laboratory report or medical record. Here’s a general outline of what you might find in a VDRL test report:
Test Results: The report will indicate the result of the VDRL test. It can be reported as positive, negative, or sometimes as an equivocal (uncertain) result.
- Positive result: This suggests either a current or past syphilis infection
- Negative result: A negative result suggests the absence of detectable syphilis antibodies in the blood.
- Equivocal result: An equivocal result means that the test results are uncertain or inconclusive. In such cases, additional testing and follow-up may be recommended to determine the presence or absence of syphilis infection.
Titer: If the VDRL test is positive, the report may include the titer of the positive reaction. The titer indicates the dilution of the serum at which a positive reaction occurs.
Interpretation: The report may include an interpretation section that provides a summary of the VDRL test result and its significance. It may also mention the need for further confirmatory testing, such as treponemal antibody tests, to validate the results.
Additional information: The report may include additional information relevant to the VDRL test, such as the date and time of the test, the laboratory performing the test, and any specific comments or notes from the healthcare provider or laboratory personnel.
Consider reading- Troponin test
How To Book
How To Book
To book a VDRL (Venereal Disease Research Laboratory) test, you can follow these general steps:
- Consult a healthcare provider
- Prescription or Referral
- Choose a testing facility
- Schedule appointment
- Follow preparation instructions
- Payment and documentation